
Since the first descriptions of the acquired immunodeficiency syndrome (AIDS) in 1981 and the subsequent discovery of the retrovirus human immunodeficiency virus (HIV) in 1983-84, both patients and the public have become very much aware of the potential for acquiring this disease through blood transfusion or therapy with blood-derived products. Yet, beginning with the introduction of a serologic blood screening test for HIV (now called HIV-1) in 1985, the risk of HIV-1 transmission through blood has been almost entirely eliminated. However, shortly after serologic screening of the general blood donor population for HIV-1 was initiated, a second retrovirus with the potential for causing AIDS, called HIV type 2 or HIV-2, was discovered (1). In this article, we will discuss the methods currently used nationwide and here at M. D. Anderson Cancer Center for screening donated blood for HIV-2 infection, as well as recent studies of HIV-2 prevalence in the blood donor populations of the United States and M. D. Anderson.
Comparison of HIV-1 and HIV-2
Both HIV-1 and HIV-2 are human
retroviruses that contain RNA as genetic material. Both infect human cells
principally through the binding of the virus outer membrane glycoprotein,
gp120, to the CD4 antigen receptor on T lymphocytes, monocytes, macrophages,
and microglia cells. Furthermore, both retroviruses can be integrated into the
human genome, and they replicate in the infected host cells using a
retrovirus-specific enzyme called the RNA reverse transcriptase. However,
nucleic acid analysis of HIV-1 and HIV-2 has shown a less than 50% homology
between the two retroviruses and suggests that HIV-2 is more closely related to
a simian retrovirus, simian immunodeficiency virus (SIV), with which it shares
about 75% sequence homology. Furthermore, though HIV-1 and HIV-2 have similar
gag (viral core) and pol (polymerase) regions, they have
relatively dissimilar env (envelope) regions. Owing to this lack of
homology in the envelope region, there is little serologic cross-reactivity of
the antibodies directed against the envelope antigens of both HIV-1 and
HIV-2.
Epidemiology
Whereas HIV-1 has spread worldwide, with over 13 million
cases of infection estimated (2), the prevalence of HIV-2 is geographically
more restricted. HIV-2 is most prevalent in West Africa, Angola, and
Mozambique; but HIV-2 infections and associated AIDS cases have also been
reported in Europe, particularly in Portugal, France, and Germany; South
America (Brazil) (3); North America (4); and India (5).
The route of HIV-2 infection in humans appears to be similar to that previously described for HIV-1. Both retrovirus infections are acquired most frequently through heterosexual and bisexual contact as well as through transfusion of contaminated blood and its by-products (6). Although HIV-1 is readily transmitted through homosexual contact, this route of transmission for HIV-2 infection has so far only been described in degrees Celsiusa few cases (7). Vertical transmission of HIV-1 and HIV-2 infection from mother to infant has been reported with transmission rates of 30% and 10% for HIV-1 and HIV-2, respectively. Thus far, no infection by HIV-2 through needle sharing has been reported in the United States (8).
Testing for HIV-2 Infections
In contrast to that of HIV-1, the
prevalence of HIV-2 infection in the United States population is believed to be
extremely low, as suggested by studies of blood donors and the screening of
patients who frequent clinics for sexually transmitted diseases (4). The
serologic diagnosis of HIV-2 infections is analogous to that of HIV-1 infection
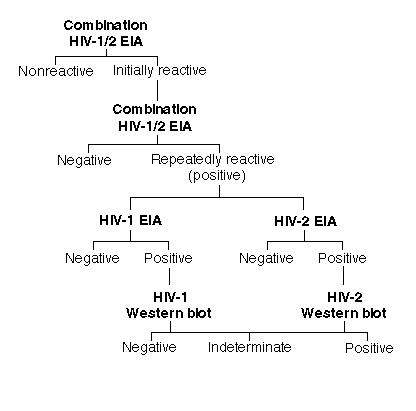
using the common test algorithm established for HIV-1 (see figure below).

Typically, an enzyme immunoassay (EIA) procedure is used to screen the blood supply for the presence of HIV-specific antibodies and involves using either a whole-virus lysate, synthetic peptides, or recombinant viral proteins as the antigen bound to plastic in a Microtiter-plate format (Genetic Systems Corporation, Redmond, WA). Each blood sample is tested as a singlet with this system; reactive samples are referred to as "initially reactive" (IR) and must be verified by repeating the testing in duplicate using the same EIA test kit. If the IR sample is found to be reactive on the second test run, the sample is labeled as "repeatedly reactive" (RR), or positive. In each RR specimen, the presence of HIV-1-specific antibody is confirmed or ruled out either by an indirect immunofluorescence assay using virus-infected target cells or by the western blot assay, which can identify antibody to specific virus antigens. More sophisticated and expensive procedures not yet approved by the U.S. Food and Drug Administration (FDA), such as in vitro culture techniques for isolating virus or detecting HIV-specific DNA or RNA by the polymerase chain reaction (PCR), are also available through a few specialized laboratories.
Although the HIV-1 EIA kits licensed by the FDA can detect HIV-2-specific antibodies in most instances (60-90%), the use of an HIV-2 EIA kit licensed by the FDA in 1990 for the sole purpose of screening the blood supply for the presence of HIV-2-specific antibody has been officially mandated by the FDA since June 1, 1992. This HIV-2 EIA kit uses a whole-virus lysate as antigen fixed on a 96-well Microtiter-plate template. More recently, the FDA licensed two additional EIA test kits that use a mixture of HIV-1 and HIV-2 virus lysates or HIV-2 (rDNA); referred to by us as "HIV-1/2 combination tests," the tests can detect antibodies against both HIV-1 and HIV-2. The FDA has approved the use of these HIV-1/2 combination tests as the initial screening assay to detect antibodies to both retroviruses in the blood supply.
Using the HIV-1/2 combination tests to screen the blood supply has eliminated the need to perform two assays, each of which was designed to detect infection with a single retrovirus. The sensitivity of the combination test system is comparable to that achieved for either HIV-1 or HIV-2 single-retrovirus EIA kits (9). Although there is an established mechanism (Figure 1) by which all specimens that are repeatedly reactive for the presence of HIV-1-specific antibody in the screening tests can be confirmed by FDA-licensed HIV-1 western blot assays, no such FDA-approved confirmatory assay for HIV-2 is currently available. Nevertheless, unlicensed immunoblot assays using synthetic antigen peptides or western blot analysis are offered through cooperative agreements with the Centers for Disease Control and Prevention (CDC) in Atlanta. The CDC criteria for scoring western blot analyses for HIV-1 and HIV-2 are as follows: HIV-1, any 2 of the following 3 bands--p24 (gag), gp41 (env), gp120/160 (env); and HIV-2, no criteria established. The World Health Organization (WHO) criteria are: HIV-1, 2 env (gp41, gp120/160) bands with or without gag (p24) or pol (p31) bands; and HIV-2, 2 env (gp36 and gp125) bands with or without gag (p26) and pol (p34) bands.
HIV-2 Infections in Blood Donors in the United States
Studies
Reported in the Literature
HIV-2 infections in the United States are
very rare. Thus far, only one confirmed case of HIV-2 infection in a blood
donor has been reported in 31,630 HIV-1 RR EIA blood samples from among
24,000,000 blood donations (10). In other recent studies, 35 of 578
HIV-1-reactive blood specimens from a pool of 869,545 blood donations were
found to be reactive using the HIV-2 EIA (11,12). None of the 35 presumed
HIV-2-reactive blood samples, however, could be confirmed as such by HIV-1- and
HIV-2-specific peptide-based assays or western blot analysis.
Based on their screening of the 24,000,000 blood donations, O'Brien et al. (4) suggested a prevalence of HIV-2-infected blood units of about 2.6 per 10,000,000 donated units. Further analysis of the data incorporating the sensitivity and specificity of the screening EIA kits indicated that less than 1% of the HIV-1/2 RR EIA specimens would be considered to be confirmed HIV-2 positive (4). This projection was recently substantiated by a CDC report of only 32 verified HIV-2 infections in the United States (4). Most of these infections were found in persons from the high-prevalence areas in West Africa or in those individuals who had had sexual contact with West Africans.
A Recent Study at M. D. Anderson
Within the last year at M. D.
Anderson, we tested blood specimens from 30,000 blood donors according to the
schema shown in Figure 1. Using an HIV-1/2 combination test kit supplied by
Genetic Systems Corporation (Redmond, WA), we detected 119 IR donors among
30,000 donors (Table 1). Upon retesting the IR specimens in duplicate, only 77
(65%) were repeatedly reactive. These 77 specimens were further tested by
HIV-1- and HIV-2-specific EIA kits for the presence of HIV-1- and
HIV-2-specific antibodies with the following results: 50 (65%) were HIV-1
positive and 14 (18%) HIV-2 positive. Subsequently, western blot testing to
confirm the presence of antibodies of both HIV-1 and HIV-2 yielded one
confirmed HIV-1 infection.
| No. of samples | |||||||
|---|---|---|---|---|---|---|---|
| Positive EIA screens | Western blot confirmation* | ||||||
| Virus | Samples tested | IR | RR | Pos | Ind | Neg | Percent prevalence | HIV-1 | 30,000 | 119 | 77 | 1 | 40 | 9 | 0.003 |
| HIV-2 | 30,000 | 119 | 77 | 0 | 1** | 13 | <0.003 |
| * | Pos, positive; Ind, indeterminate; Neg, negative. |
| ** | Initially indeterminate. |
In another case, an HIV-2 RR serum showed faint, suspicious bands, which required that a repeat specimen be obtained to rule out HIV-2 infection by western blot and PCR analysis; but the repeat testing did not confirm the presence of HIV-2. Among 49 HIV-1 RR specimens subjected to western blot analysis, 40 exhibited a variety of atypical bands, which we interpreted as "indeterminate" according to the CDC classification for western blot evaluation. The nine remaining HIV-1 RR specimens were negative by western blot analysis.
The results of our study indicate an HIV-2 prevalence of less than 0.003% in M. D. Anderson's blood donor population. These results agree with those of the aforementioned studies in indicating an extremely low HIV-2 prevalence in the United States blood donor pool. Furthermore, we experienced a not insignificant loss to our blood donor program of 77 whole blood or platelet donors through false-positive serologic HIV-1/2 tests.
Conclusion
Though there is a real danger that HIV-2 may be spread
through blood transfusion and hemotherapy, studies by us and by others outside
M. D. Anderson show that the prevalence of HIV-2 infection in blood donors at
M. D. Anderson and in the United States is still extremely low. However, in the
atmosphere of concern that surrounds both patients and the public, FDA-licensed
kits for screening blood for HIV-2, and in particular the recently licensed
HIV-1/2 combination tests, have helped and will help to ensure the safety of
the blood supply. Such kits have also eliminated the need for administering
separate tests for HIV-1 and HIV-2. Yet, despite the very low prevalence of
HIV-2 infection in the United States and in M. D. Anderson's blood donor
population, it is important that the specificity of HIV-1/2 combination tests
be improved significantly to prevent unnecessary exclusion of donors as
a consequence of false-positive test results.
Nevertheless, the present sensitivity of these assays should permit the identification of HIV-2-contaminated blood and blood by-products in the nation's blood supply. Unlike in our previous experience in 1981 with HIV-1, when there was no test available for screening the blood supply against HIV-1, the measures we have discussed above for screening against HIV-2 have contributed to a proactive response by the FDA and the transfusion and blood-banking community to the threat of HIV-2 contamination of the blood supply.
References
Newsletter homepage URL: http://www.mdacc.tmc.edu/~citm/