
The human T-lymphotropic virus type I (HTLV-I) and type II (HTLV-II) were isolated and known several years before the human immunodeficiency virus (HIV-1). Yet, less attention was initially paid to them and their transmission through blood products than to HIV. Only in November 1988, more than 3 years after HIV screening was introduced, did the U.S. Food and Drug Administration (FDA) recommend that the U.S. blood supply be screened for HTLV-I.
The delay in screening for HTLV may have been due in part to the lack of convincing evidence that HTLV-I transmission would invariably cause disease, as observed for HIV. For instance, adult T-cell leukemia/lymphoma, a disease known to be associated with HTLV-I, presumably takes several decades to manifest itself and occurs in less than 2-4% of infected individuals (1). Furthermore, HTLV-I-associated demyelinating disease has been reported in only a few cases; in fact, the connection between virus and neurological disease in such cases is still obscure. HTLV-II-associated disease is even less proven, though a few such cases of the rare T-cell hairy cell leukemia have been reported. Nevertheless, it is becoming clear that HTLV-I and HTLV-II can cause disease.
This article describes the methods currently used to screen against HTLV-I/II in the blood supply in the United States and at M. D. Anderson Cancer Center. It also presents data on HTLV seroprevalence in these blood donor populations and in patients at M. D. Anderson.
Comparison of HTLV-I and HTLV-II
HTLV-I and HTLV-II are human,
single-stranded RNA retroviruses of the so-called C type originally described
by Gallo's group at the National Cancer Institute in 1980 and 1982,
respectively (2,3). Both viruses were isolated from patients with T-cell
leukemias/lymphomas and have been shown to infect human CD4+ and
CD8+ lymphocytes (4). They contain no oncogenes but can transform
human lymphocytes in vitro. They differ from all known animal retroviruses in
their nucleic acid composition and hybridization and in their structural
proteins. The viruses consist of essentially four genes that code for
structural and regulatory proteins: gag, pol, env, and
px. Being enveloped viruses, they can integrate their genomes into human
DNA via reverse transcription of RNA into DNA.
HTLV-I and HTLV-II are closely related: they share about 60% homology in their nucleotide sequences, have very similar structural and envelope proteins, and elicit similar immune responses in humans (5). In fact, it is very difficult to differentiate serologically between the HTLV-I and HTLV-II antibodies produced by infected humans or to determine the etiologic agent of the infection because (a) the antigens are so similar and (b) the generated antibodies are cross-reactive. The best and most convenient method of unequivocal discrimination is the polymerase chain reaction (PCR), although discrimination by western blot (6) or by reactivity to synthetic peptide antigens (7) has been tried.
Epidemiology
Infections of humans by HTLV are thought to be life
long. They also appear to occur worldwide, having been reported in North and
South America, the Caribbean, Europe, Africa, and Asia. They are endemic in
certain parts of Africa, Japan, the Caribbean, and Europe (8,9).
HTLV-I-associated leukemias/lymphomas have been observed mainly in Japan and
Africa and neurological disorders such as tropical spastic paraparesis (TSP) or
HTLV-I-associated myelopathy (HAM) predominantly in Japan and the Caribbean
(8,9).
Routes of infection include transfusion, sharing of needles or syringes with infected individuals, sexual contact, and breast feeding; transplacental transmission is also suspected (10-17). Cellular blood products are the main source of transfusion-associated HTLV transmission, whereas fresh frozen plasma, cryoprecipitate, or coagulation factor concentrates appear not to cause infection (18,19).
Early antibody screening tests did not reliably distinguish between HTLV-I and HTLV-II infections because both viruses are closely related. But recently, increased use of PCR methodology has shown that significant numbers of HTLV-antibody-positive individuals are actually infected by HTLV-II and not by HTLV-I, as previously assumed (20,21). This has been demonstrated particularly for i.v. drug users in the United States (14,22). Furthermore, distinct infections by both HTLV-I and HTLV-II have recently been described in Japanese blood donors (23).
Testing for HTLV-I/II
The prevalence of HTLV infection in the U.S.
population is not known. But among volunteer whole blood donors, several
studies have shown a seroprevalence of about 0.025-0.030% (24,25) and among a
group of U.S. paid plasma donors, a rate as high as 0.3% (26). Thus, the need
for donor screening is evident.
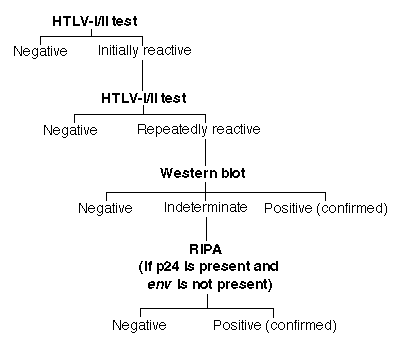
Blood donor screening in the United States is done mainly with enzyme immunoassays (EIAs) approved and recommended by the FDA. Other testing methods, not FDA licensed, include agglutination assays, which are widely used in Japan (27), and more cumbersome indirect immunofluorescence tests (2,28). EIAs allow fast and efficient screening for the HTLV antibody within 2-3 h: serum samples are reacted with either synthetic peptides, recombinant viral proteins, or viral lysates bound as antigen to plastic Microtiter plates. At M. D. Anderson, the schema for blood screening for HTLV-I/II is as follows. Each specimen is tested first as a singlet. If a sample is initially reactive (IR), the test is repeated in duplicate. If the IR sample is reactive in either of the repeat tests, it is designated "repeatably reactive" (RR), or positive. Each RR specimen is subjected to further confirmatory testing usually by western blot and, if antibody to p24 is present and antibody to the envelope antigen (usually gp46) is not detected, by radioimmunoprecipitation assay (RIPA) (see figure below). These confirmatory tests either identify or rule out the presence of antibodies to distinct viral antigens such as gag (p19 or p24) and env (gp 21e [recombinant], gp46, or gp61/68). The CDC and WHO criteria for interpretation of positive HTLV western blot and RIPA tests (34) are as follows: HTLV-1, presence of p24 and any of 3 env bands [gp21e (a recombinant antigen not originally included in the criteria), gp 46, or gp61/68]; and HTLV-II, no criteria established yet.

Other more sophisticated tests based on PCR technology, on nucleic acid analysis, or on hybridization methods are not yet FDA approved and are performed only in very specialized laboratories. Another very laborious method involves coculturing cells suspected of harboring HTLV with noninfected cells in order to stimulate production and expression of HTLV. The expressed viral antigens can then be detected by immunofluorescence using an antibody such as anti-p19 (2).
The commercially available EIA test kits were originally designed to exclude HTLV-I-antibody-positive blood products from the blood supply, to prevent patients from being infected with HTLV-I, and ultimately to prevent the development of adult T-cell leukemias or lymphomas. However, recent reports of HTLV-II transmission via blood transfusion (19) and HTLV-II-associated hematologic (29,30) and neuromuscular diseases (31-33) have intensified the efforts of blood banks to protect patients from HTLV-II-contaminated blood products as well, by improved donor screening.
Recent Results in M. D. Anderson and U.S. Blood Donors
Using
commercially available EIA test kits, we recently screened a total of 39,908
volunteer blood donations made at M. D. Anderson between September 1, 1993, and
August 31, 1994. The results are summarized in Table 1.
| No. samples | ||
|---|---|---|
| EIA results* | ||
| IR | 158 | 0.4% |
| RR | 105 | 0.26% |
| Confirmatory test results in RR specimens** | ||
| Negative | 33 | 0.08% |
| Indeterminate | 66 | 0.17% |
| Positive | 6 | 0.015% |
| * | 39,908 blood donations made between September 1, 1993, and August 31, 1994, were screened by EIA. |
| ** | 105 RR specimens were subjected to confirmatory testing (western blot and/or RIPA). Of the six specimens positive by western blot, two required further confirmation by RIPA. |
Of the 39,908 specimens, 158 (0.4%) were IR and 105 (0.26%) were RR. Confirmatory testing of the 105 RR specimens showed 66 (0.17%) to be indeterminate and only six (0.015%) seropositive: seropositivity was confirmed by western blot in four cases and western blot and RIPA in two cases. Our test specificity of 99.8% compares very well with the estimated specificities of 99.3-99.9% reported by the Public Health Service Working Group (PHSWG) (34). However, the predictive value is quite low, as we could confirm positivity in only six of 103 (6%) of the RR specimens; conversely, PHSWG found that only 10 of 68 (15%) were confirmed positive among more than 5,000 normal U.S. blood donors from nonendemic areas (34).
The seroprevalence of 0.015% among M. D. Anderson blood donors is slightly lower than the 0.025% reported by Williams et al. (24) for about 40,000 U.S. blood donors from eight different blood centers. Similar results were obtained from 267,650 blood donors in the U.S. Armed Forces: 72 donors (0.027%) were confirmed positive for HTLV antibodies (25). A somewhat higher seroprevalence of 0.08% was reported for 18,257 whole blood donors from the greater New York City area (35).
In comparison, a seroprevalence up to 10 times higher was observed in paid plasma donors from five different parts of the United States: 19 of 6,286 (0.3%) donors were positive (26). However, when 154 French and 25 U.S. hemophiliacs who were regularly receiving noninactivated plasma products were tested, none was HTLV seropositive (26).
Recently, Hjelle et al. (36) reported that of 61,752 donor blood samples, 17 tested HTLV positive by EIA and were confirmed by western blot; however, by testing 178 samples that had an absorbance reading of greater than 50% of the EIA cutoff, they found that 11 additional specimens were confirmed by both western blot and PCR. This suggests that the current EIA-based testing might miss up to 40% of HTLV-positive donors.
Busch and coworkers (37) found that of 994 HTLV RR specimens, only 410 samples could be confirmed on further testing. Three were false-positive samples obtained in the first year of testing. However, of the remaining 407 serologically confirmed specimens, 403 were shown by PCR to be infected by HTLV-I or HTLV-II (123 for HTLV-I and 280 for HTLV-II). Of 426 indeterminate specimens, six (1.4%) turned out to be positive by PCR: one was positive for HTLV-I and five for HTLV-II.
In another study, Lee et al. (38) tested 480,000 blood donors in five different regions of the United States and determined the relative seroprevalence of HTLV-I versus HTLV-II infections. Among 207 HTLV-I/II-seropositive donors, 65 were further investigated by PCR: 34 specimens (52%) showed HTLV-II infection, 28 (43%) showed HTLV-I infection, and 3 (5%) were uninformative. Further interviews of donors demonstrated that the main risk factor for HTLV-I infection was donor origin, i.e., geographic region, whereas the main risk factor for HTLV-II infection appeared to be i.v. drug use.
Results of M. D. Anderson Patient Screening
From September 1, 1993,
to August 31, 1994, we tested a total of 867 cancer patients for HTLV
antibodies (see Table 2). Samples from eight (0.9%) patients were RR for HTLV
antibodies. Further confirmatory testing by western blot and RIPA, when
indicated, revealed only one (0.1%) patient with previous HTLV exposure or
infection, five (0.58%) patients indeterminate for antibodies, and two (0.23%)
patients negative. Three patients had hematologic malignancies. Two patients
had breast cancer. One patient with Mycosis fungoides showed only
antibodies against gp21e. Because our results were obtained retrospectively, we
could not assess risk factors. No further DNA-based testing was performed on
specimens deemed indeterminate.
| Western blot | |||||||
|---|---|---|---|---|---|---|---|
| Diagnosis | Sex | Age (years) | Result | Bands present | RIPA result | Final result | |
| AML | F | 63 | Ind | p19, p24 | Pos | Pos | |
| Breast carcinoma | F | 40 | Ind | p19, p28, p53 | ND | Ind | |
| B-CLL | M | 50 | Neg | None | ND | Neg | |
| T-cell malignancy (Mycosis fungoides) | M | 79 | Ind | gp21e | ND | Ind | |
| Breast carcinoma | F | 35 | Ind | p19, p28 | ND | Ind | |
| Cervical carcinoma | F | 36 | Ind | p19, p26, p28 | ND | Ind | |
| Squamous carcinoma, tongue | F | 41 | Neg | None | ND | Neg | |
| Prostate carcinoma | M | 60 | Ind | p19 | ND | Ind | |
* AML, acute myelogenous leukemia; B-CLL, B-cell lymphocytic leukemia; F, female; M, male; ND, not done; Ind, indeterminate; Neg, negative; Pos, positive.
Conclusion
The introduction of blood donor screening for HTLV
antibodies in November 1988 significantly decreased the transmission of
HTLV-I/II through cellular blood components. As a consequence, patients have
been and should continue to be protected from developing HTLV-associated T-cell
leukemia/lymphoma and neurodegenerative disease resulting from blood
transfusion. The prevention of HTLV infection by blood donor screening has
already been shown to be effective in Japan (12,28). In the United States, the
low seroprevalence of HTLV in the blood donor population (2.5 of every 10,000
donors are infected by HTLV), the limited survival (10-14 days) of the HTLV
provirus in stored red blood cell concentrates (15), and the requirement that
sufficient viable infection-transmitting lymphocytes be present for disease to
occur may have prevented a larger epidemic. Also, the absence of lymphocytes in
the coagulation factor concentrates and cryoprecipitates used to treat
hemophiliacs has spared these patients infection.
Nevertheless, the rate of HTLV-II infection in i.v. drug users is suspected to be increasing. This is of particular concern because the current serologic tests are more effective at keeping HTLV-I-seropositive blood than HTLV-II-seropositive blood out of the donor pool. Therefore, the methods for detecting HTLV-II-infected donors must be improved, and a simpler, faster assay for confirming HTLV-II infection, thus allowing more effective donor counseling, must be developed.
The data on HTLV seroprevalence among patients at M. D. Anderson are very preliminary. Patients with indeterminate test results would require retesting. Although the seroprevalence among our patients is 0.1% and about six times higher than in our normal blood donor population, our limited number of subjects does not allow us to draw valid conclusions about this group, particularly since we did not assess risk factors, e.g., transfusion history, place of origin, i.v. drug use. However, in general, testing of patients is indicated whenever risk factors are known and HTLV-associated diseases are suspected.
References
Newsletter homepage URL: http://www.mdacc.tmc.edu/~citm/